ADR coverage organization
Product Description
ADR coverage organization
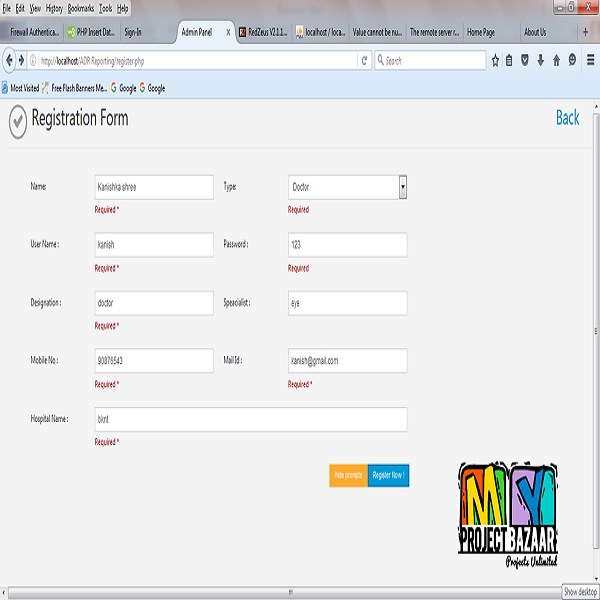
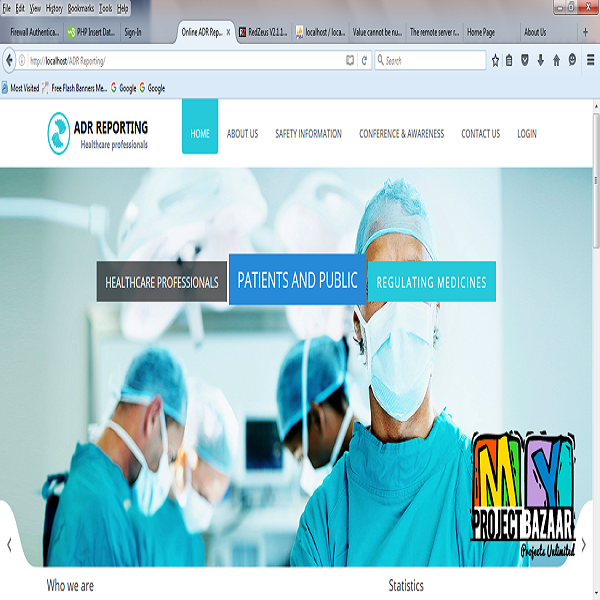
Abstract—New drugs are regularly introduced for treatment, diagnosis or prevention of diseases. Adverse drug reactions < Final Year Projects 2016 > can be seen in clinical practice with both new as well as marketed medicines. Spontaneous reporting of Adverse drug reactions is commonly practiced method for monitoring of Adverse drug reactions. Healthcare practitioners have an important role in pharma covigilance and Adverse drug reactions reporting. Many studies have been conducted to understand the knowledge, attitude and practice of Adverse drug reactions reporting among healthcare practitioners in India. The population surveyed in these studied ranged between 90 -1200. One large study4 included population of 1200 physicians across India out of which 1000 were contacted for study participation.
Including Packages
Our Specialization
Support Service
Statistical Report

satisfied customers
3,589
Freelance projects
983
sales on Site
11,021
developers
175+Additional Information
| Domains | |
|---|---|
| Programming Language |